The TP-II study is a multicenter, randomized phase II trial comparing 2 neoadjuvant de-escalation treatments in HR-positive/ERBB2-positive EBC that palleos healthcare conducts as sponsor. Due to outstanding recruitment, study treatment of the last patient was completed earlier than originally planned in July 2020. In the TP-II trial, an excellent pCR rate after 12 weeks of paclitaxel plus trastuzumab and pertuzumab treatment could be demonstrated that was clearly superior to the pCR rate after endocrine therapy plus trastuzumab and pertuzumab. The main results of this study have been recently published in the renowned peer review journal JAMA Oncology.
Study design
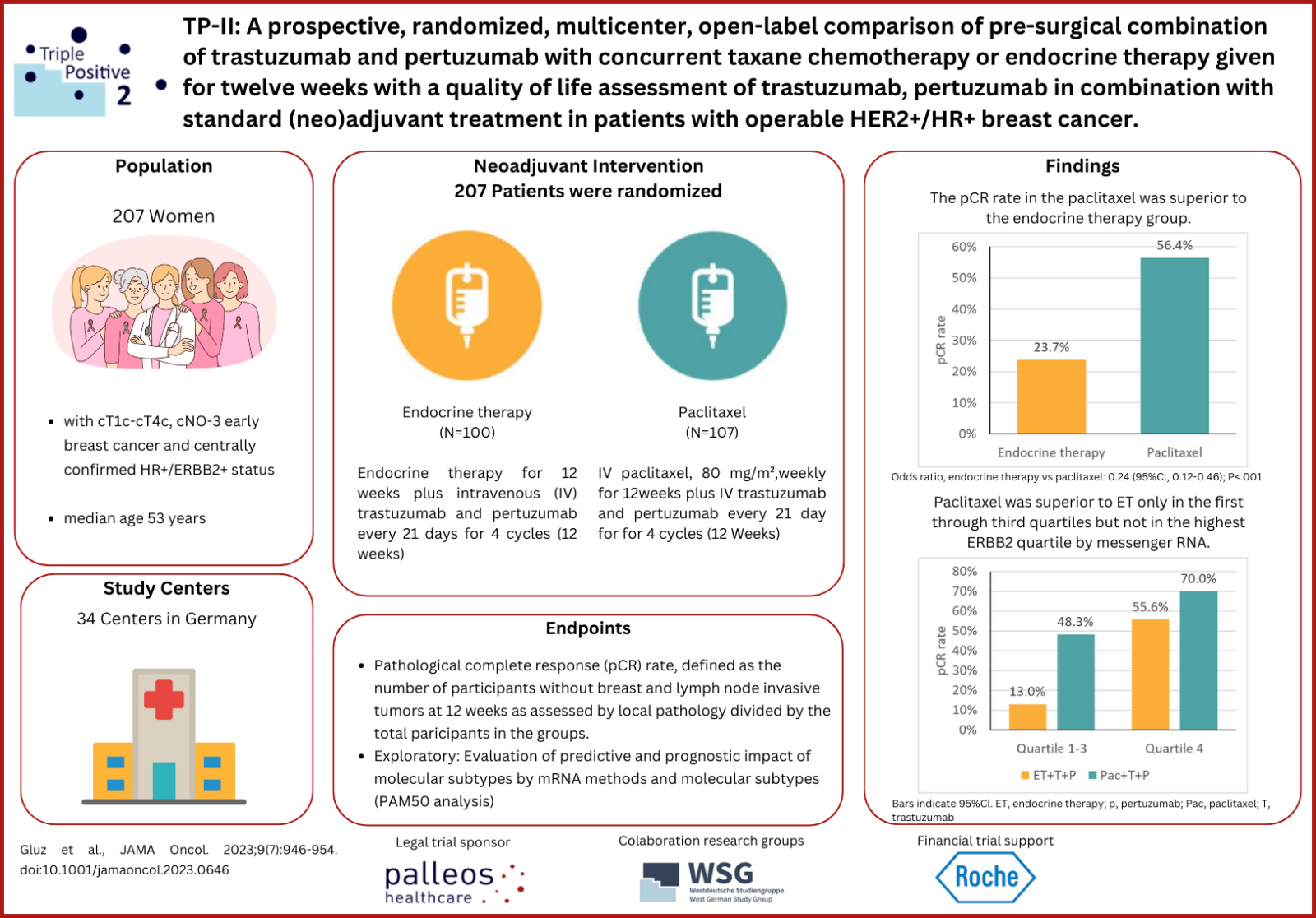
The TP-II study (NCT03272477) is a multicenter, randomized phase IIa study comparing 2 neoadjuvant de-escalation treatments in highly heterogeneous hormone receptor (HR)-positive/ERBB2-positive early breast cancer (EBC) which is conducted in Germany. Patients were randomized (1:1) to arm A receiving endocrine therapy (ET) or to arm B receiving de-escalated standard taxane chemotherapy, both in combination with pertuzumab and trastuzumab (dual ERBB2 blockade) for 12 weeks. Depending on the prior neodajuvant treatment and the pathological examinations of their tumor biopsy or surgical sample, patients received adjuvant treatment after surgery. Patients randomized to arm A were recommended to receive complete standard adjuvant chemotherapy treatment, preferentially paclitaxel weekly and 4 x EC (epirubicine and cyclophosphamide, 4 cycles). Trastuzumab and pertuzumab were allowed together with paclitaxel. Patients randomized to arm B were recommended to be treated by anthracycline-based chemotherapy (e.g., EC x 4). Following the treatment of adjuvant chemotherapy, all patients received trastuzumab and pertuzumab and endocrine therapy and patients should complete anti-ERBB2 treatment over 40 weeks (14 cycles). Total duration of dual ERBB2 blockade (neoadjuvant and adjuvant) was over 52 weeks (18 cycles). The omission of further adjuvant chemotherapy was allowed only in cases with pathological complete response (pCR; ypT0/is, ypN0) at discretion of the investigator.
Outcomes
A total of 207 patients were randomized (median [range] age, 53 [25-83] years) at 34 study sites between September 2017 and March 2019 (100 in arm A vs. 107 in arm B). Study treatment of the last patient was completed in July 2020. The primary endpoint was pCR rate after 12 weeks of neoadjuvant treatment. Secondary end points include safety, translational research, and health-related quality of life. The treatment with paclitaxel plus trastuzumab and pertuzumab (arm B) was associated with a superior pathological complete response (pCR) rate of 56% vs 24% after very well-tolerated endocrine therapy plus trastuzumab and pertuzumab (arm A). Immunohistochemical ERBB2 score of 3 or higher and ERBB2-enriched subtype (analysis of microarray (PAM50) subtype predictor including ERBB2 expression) were both independent predictors for pCR in both arms. Paclitaxel was superior to ET only in the first through third quartiles but not in the highest ERBB2 quartile determined by messenger RNA. In contrast to arm B with the paclitaxel plus trastuzumab and pertuzumab, no decrease in health-related quality of life after 12 weeks was observed in the ET plus trastuzumab and pertuzumab arm A. Further secondary endpoints will be analyzed after completion of the study (estimated for end of February 2024).
Conclusion
High pCR rates after de-escalated regimens indicate a substantial efficacy of mono-chemotherapy regimen in combination with dual ERBB2 blockade in patients with mostly stage II hormone receptor–positive/ERBB2-positive EBC.
Reference:
Gluz O, et al. Efficacy of endocrine therapy plus Trastuzumab and Pertuzumab vs de-escalated chemotherapy in patients with hormone receptor-positive/ERBB2-positive early breast cancer: The neoadjuvant WSG-TP-II randomized clinical trial. JAMA Oncol. 2023 Jul 1;9(7):946-954. doi: 10.1001/jamaoncol.2023.0646. PMID: 37166817; PMCID: PMC10176180.