End-to-End Support
We support our clients over the full development lifecycle
palleos healthcare is a full-service Clinical Research Organization (CRO) for the development of healthcare products, active in central and eastern Europe.

19 years experience
Medicinal Products
Medical Devices
Consulting
End-to-End Support
We support our clients over the full development lifecycle
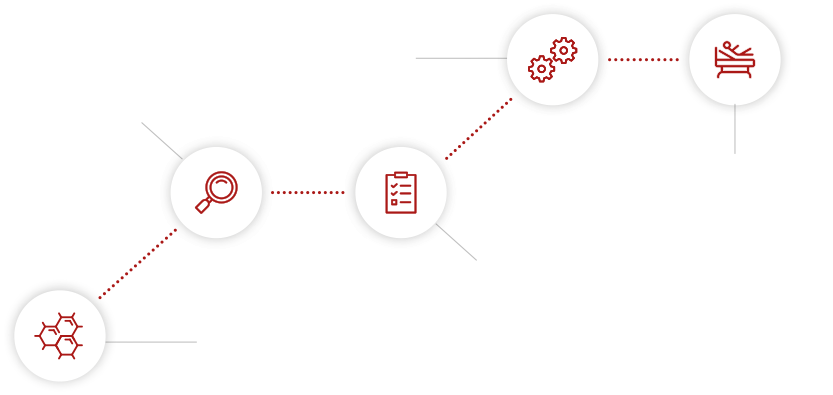
palleos healthcare’s service segments cover the entire product development life cycle
Our stepwise process identifies and prioritizes a healthcare product’s target indications with precision. This process cuts no regulatory corners, but we balance it in a resource-efficient manner.
read more...We aid our clients in crafting and maintaining a dynamic TPP/Intended Use document. This living document is meticulously designed to define and communicate indications, market niches, and development pathways. We use it as a guiding tool to monitor progress and navigate product development seamlessly towards approval.
read more...At palleos healthcare, we play a key role in developing your comprehensive Development Plan. This detailed summary encapsulates the entire preclinical and clinical research strategy, outlining the critical path for your healthcare product’s development toward market authorization. Trust us to guide you through this crucial process.
read more...We offer a full suite of services for executing Phase I-IV clinical trials, Non-Interventional Studies (NIS), registry-based studies and medical device studies (incl. IVD). From initial planning to study closure, our comprehensive support includes: Project Management, Regulatory Affairs, Monitoring, Medical Monitoring, Pharmaco- and Materiovigilance, Data Management, Statistics, Medical Writing and Quality Management. We always prioritize the unique needs of every stakeholder, ensuring a tailored approach to each project.
read more...We recognize the growing importance of non-interventional trials. These provide valuable information on product use after market approval during routine clinical application. Tailoring our approach to our customers’ individual preferences and needs, we oversee the entire study process - from registration to final evaluation - ensuring a seamless and comprehensive service for you.
read more...palleos healthcare is a full-service Clinical Research Organization (CRO).
We offer a full suite of services for executing Phase I-IV clinical trials, Non-Interventional Studies (NIS), registry-based studies and medical device studies (incl. IVD).
From initial planning to study closure, our comprehensive support includes Project Management, Regulatory Affairs, Monitoring, Medical Monitoring, Pharmaco- and Materiovigilance, Data Management, Statistics, Medical Writing and Quality Management.
We prioritize the unique needs of every stakeholder, ensuring a tailored approach to each project.
Get in touch with us!
Interested in partnering with palleos healthcare or have questions? Submit the form below for a prompt response. Thank you for considering us!